Introduction:
Cytogenomic abnormalities are integral to disease classification, risk stratification, and treatment plans for patients with myeloid neoplasms (MNs). Standard cytogenetic (SCG) techniques, including chromosomal analysis (karyotyping) and fluorescence in situ hybridization (FISH), both carry limitations in detecting certain cytogenomic abnormalities, such as chromoanagenesis, a genomic catastrophe that results in extensive chromosomal rearrangements and copy number alterations. Optical genomic mapping (OGM) is a novel, non-sequencing technique that allows for high-resolution genome wide detection of all classes of structural variants (SVs) and copy number variants (CNVs). This study aimed to investigate cytogenomic alterations across different types of MNs and evaluate the added value of OGM alongside SCG in this context.
Materials and methods:
OGM was performed using Saphyr from Bionano Genomics (San Diego, USA) on blood or bone marrow from 146 patients diagnosed with various types of MNs over a 9-month period. All patients had karyotype and/or FISH results available. Cases were considered concordant if OGM and SCG both showed normal results or essentially identical cytogenetic abnormalities. Cases were summarized under ‘SCG only’ or ‘OGM only’ if abnormalities were detected by only one assay. Only Tier 1 (pathogenic) and Tier 2 (likely pathogenic) variants were included for a comparison purpose. Abnormalities leading to changes in disease classification, risk stratification, and/or potential patient management were considered as clinically significant. Of note: AML with PML::RARA, RUNX1T1::RUNX1 or MYH11::CBFB rearrangement identified during the fast FISH screen were not included in this study.
Results:
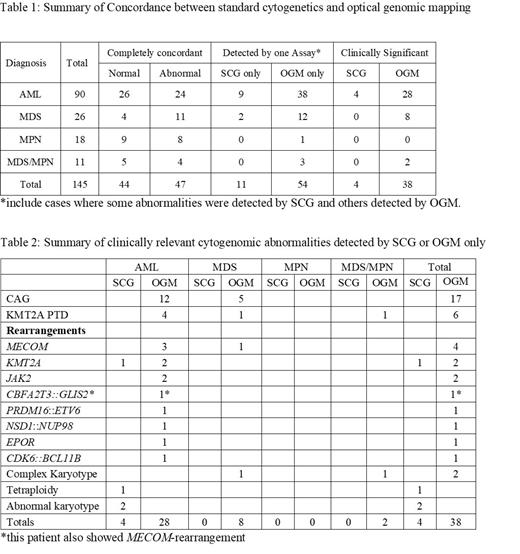
This study cohort included 90 AML, 26 myelodysplastic syndrome (MDS), 18 myeloproliferative neoplasms (MPN) and 11 MDS/MPN. Overall, 44 (30%) patients showed normal results and 47 (32%) patients showed completely matched cytogenomic abnormalities by OGM and SCG, the remaining 54 (37%) cases showed abnormalities detected by only one assay. Within these 54 cases there were 65 abnormalities detected by only one modality, 11 by SCG only and 54 by OGM only ( Table 1). Of them, 42 were clinically significant: 4 detected by SCG only (in 4 patients) and 38 by OGM only (in 37 patients) ( Table 2). The abnormalities that were detected by SCG but not by OGM were mainly clones or subclones of small (<20%) clonal size, which were below the limit of OGM detection, except one case with tetraploidy in a large clone (65%). The abnormalities detected by OGM but not by SCG mainly included chromoanagenesis (n=17), KMT2A partial tandem duplications (PTD, n=6), chromosomal /gene rearrangement (-R), thirteen being clinically significant: MECOM-R(n=4) , KMT2A-R(n=2) , JAK2-R (n=2) and 5 others in a single case each. Among the various types of MNs, OGM provided additional clinically relevant cytogenomic information in 28 (31%) patients with AML, 8 (31%) patients with MDS, 2 (18%) patients with MDS/MPN, and none (0%) with MPN.
Conclusions:
Overall, the cytogenomic results completely matched between OGM and SCG in 62% of patients with MNs. The high concordance results seen in MPN suggests OGM may not provide significant additional insight in this setting. OGM identified additional clinically relevant abnormalities in approximately 26% of patients in this cohort. The abnormalities detected by OGM, notably included chromoanagenesis and KMT2A PTDs, were found to occur more frequently in AML and MDS. These abnormalities, undetectable by SCG, are crucial to identify due to their association with significantly poorer prognosis. Additionally, certain abnormalities such as MECOM-R, KMT2A-R, and JAK2-R could potentially guide different therapeutic strategies.
Disclosures
Ravandi:Prelude: Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Biomea fusion: Honoraria, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Xencor: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding. Kantarjian:Abbvie: Consultancy, Honoraria; Ascentage Pharma Group: Honoraria; Amgen: Honoraria; Astellas Pharma: Honoraria; AstraZeneca/MedImmune: Honoraria; Daiichih-Sankyo (Inst): Honoraria, Research Funding; Immunogen (Inst): Honoraria, Research Funding; Ipsen: Honoraria; Jazz Pharmaceuticals (Inst): Honoraria, Research Funding; KAHR Medical: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Precision Biosciences: Honoraria; Shenzhen Target Rx: Honoraria; Taiho Pharmaceutical: Honoraria; Abbvie (Inst): Research Funding; Amgen (Inst): Research Funding; Ascentage Pharma (Inst): Research Funding; Bristol-Myers Squibb (Inst): Research Funding; Novartis (Inst): Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal